2For a gas formed by 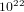 molecules which has volume equal to 1 lt in room
temperature and atmospheric pressure, the average velocity of its molecules is
molecules which has volume equal to 1 lt in room
temperature and atmospheric pressure, the average velocity of its molecules is 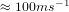 .
This means that the typical de Broglie wavelength of the molecules is
.
This means that the typical de Broglie wavelength of the molecules is 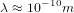 . If we
estimate that the volume occupied by each molecule is of order
. If we
estimate that the volume occupied by each molecule is of order 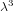 , then the number of states
that each molecule can be is
, then the number of states
that each molecule can be is 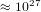 . Therefore the system can be in
. Therefore the system can be in 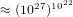 different states. If we assume that on the average the molecules collide
different states. If we assume that on the average the molecules collide 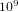 times per
second, then we have
times per
second, then we have 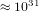 changes of states per second. In order that the system
visits all possible states, the time needed is
changes of states per second. In order that the system
visits all possible states, the time needed is 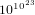 times the age of the universe
[4].
times the age of the universe
[4].